Describe the Process by Which Chlorofluorocarbons Deplete the Ozone Layer
Such atoms react with and kill ozone. Under the original Montreal Protocol agreement 1987 developed countries were required to begin phasing out CFCs in 1993 and achieve a 20 reduction relative to 1986.

Ozone Layer Depletion Is An Environmental Problem Reading Comprehension Esl Worksheet By Shmiskeen Ozone Layer Reading Comprehension Environmental Problem
ClO O Cl O2 However it is not just CFCs that can deplete the ozone layer.

. Chlorofluorocarbon and hydrochlorofluorocarbon refrigerants have been widely used in traditional cooling systems. Up to 24 cash back Ozone depletion is the term commonly used to describe the thinning of the ozone layer in the stratosphere. For example the marine ecosystem the whole.
Rowland a professor of chemistry at the University of California Irvine and Molina a postdoctoral fellow in Rowlands laboratory had shown that chlorofluorocarbonsCFCscould destroy ozone a molecule made up of three oxygen atoms O 3 in Earths stratosphere. See Ozone Depleting Substance. The total amount of effective halogens chlorine and bromine in the stratosphere can be calculated and are known as the equivalent effective stratospheric chlorine EESC.
The process of ozone depletion Each chlorine atom is capable of destroying or basically turning ozone into oxygen and which again doesnt have the same capability as ozone does in shielding us from the UV radiation. When the scientists reported their findings in 1974 CFCs were in wide. Sherwood Rowland and Mario J.
The Montreal Protocol finalized in 1987 is a global agreement to protect the stratospheric ozone layer by phasing out the production and consumption of ozone-depleting substances ODS. The Montreal Protocol on Substances that Deplete the Ozone Layer The original Montreal Protocol signed in 1987 was the first step in international efforts to protect stratospheric ozone. The compounds containing CFCs chlorofluorocarbons are mainly responsible for ozone layer depletion as these compounds react with ozone in the presence of ultraviolet rays to form oxygen molecule and thus destroying ozone.
As elevation increases the stratosphere gets warmer. Molina discovered that chlorofluorocarbons CFCs could deplete Earths atmospheric ozone layer which blocks the suns damaging ultraviolet rays. The stratospheric ozone depletion process begins with the emission of halogen source gases at Earths surface and ends when reactive halogen gases are removed by rain and snow in the troposphere and deposited on Earths surface.
Chlorofluorocarbons and Ozone Depletion At the University of California Irvine F. The ozone depletion reactions are. Ozone Depetion is caused by the release of Chlorofluorocarbons CFCs hydroflurocarbons HCFCs and other ozone-depleting substances ODS which are popularly used as refrigerants insulating foams and solvents.
The stratosphere gets its name because it is stratified or layered. Due to the Cl regeneration it only takes one chlorine radical to destroy a lot of Ozone molecules. As shown above Cl radicals are formed by the break down of CFCs by UV radiation these radicals are homogeneous catalysts theyre in the same phase as the ozone molecules gas.
The stratosphere increases in warmth with elevation because ozone. Effects of Ozone depletion Damage to human health. Ecosystems Ozone depletion has an adverse effect on the ecosystems on the earth.
Chlorofluorocarbons CFCs or chlorofluorocarbons are the primary cause of ozone layer loss. Ozone is naturally formed in the atmosphere. In the stratosphere the reactive halogen gases namely chlorine monoxide ClO and bromine monoxide BrO destroy ozone.
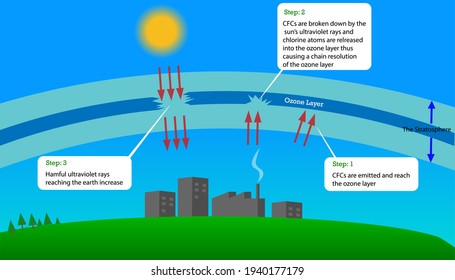
In 1974 chlorofluorocarbons CFCs were identified as playing a major role in depleting the stratospheric ozone layer a process which could potentially result in increasing levels of UVB radiation. Most of these gases accumulate in the lower atmos-. The UV radiations break down chlorofluorocarbon molecules in the stratosphere releasing chlorine atoms.
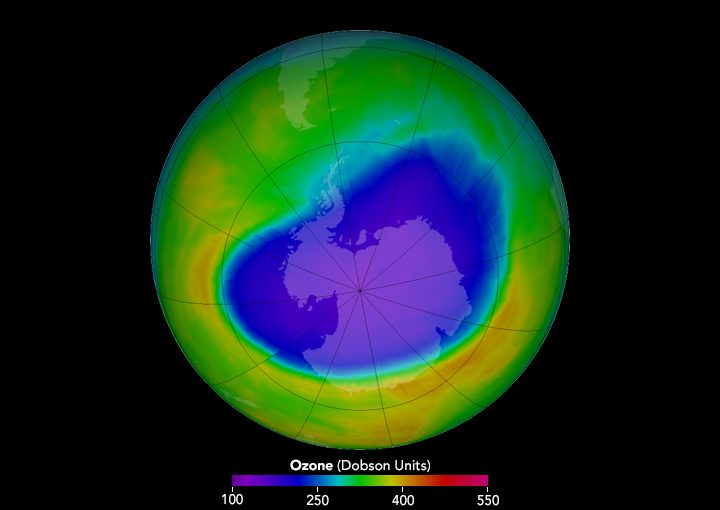
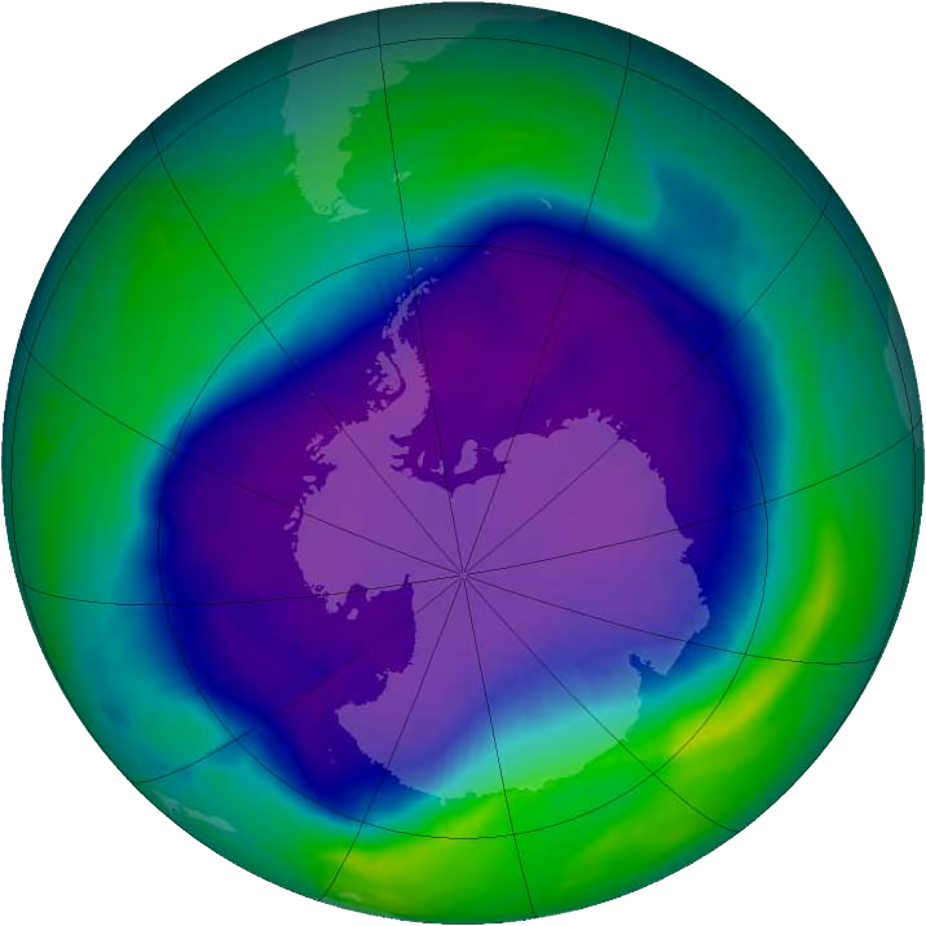
The primary cause of ozone-layer depletion is chlorofluorocarbons or CFCs. In 1985 Joseph Farman reported on the formation of an ozone hole actually a large-scale thinning of the ozone layer that develops over Antarctica every austral spring. One chlorine molecule has the capability to break down thousands of ozone molecules.
Extinction will rapidly increase by the cause of ozone depletion. These refrigerants accelerate the depletion of the Earths ozone layer. Gaseous CFCs can deplete the ozone layer when they slowly rise into the stratosphere are broken down by strong ultraviolet radiation release chlorine atoms and then react with ozone molecules.
Chlorine and bromine are known to deplete the ozone layer at supersonic speeds. They do this by simply stripping off an atom from the ozone molecule. The chlorofluorocarbon molecules in the stratosphere are broken down by ultraviolet radiation and chlorine atoms are released.
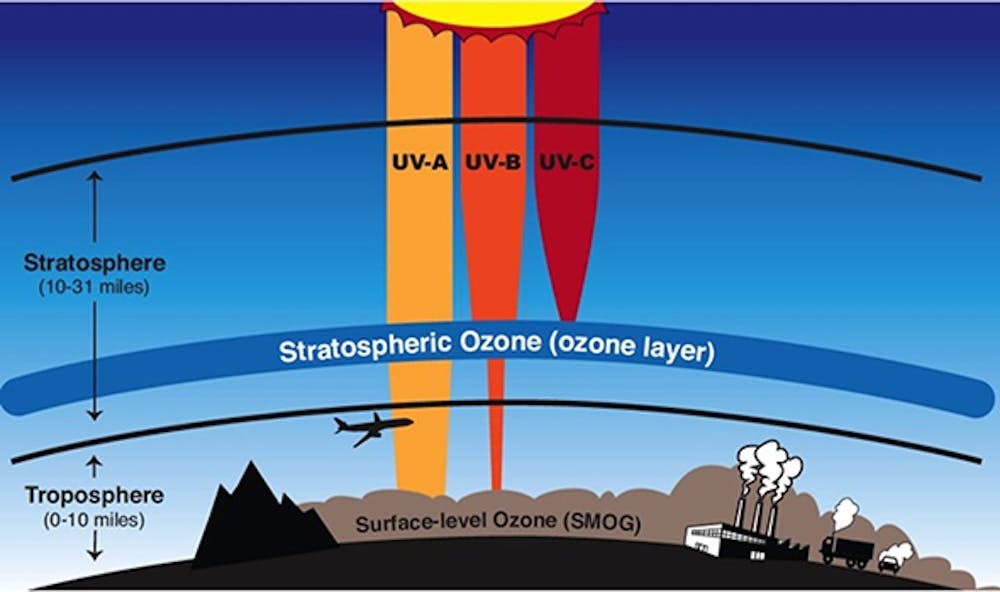
Solvents spray aerosols refrigerators air conditioners and other appliances emit these. The ozone layer is one layer of the stratosphere the second layer of the Earths atmosphereThe stratosphere is the mass of protective gases clinging to our planet. These atoms react with and destroy ozone.
Cl O3 ClO O2 When chlorine monoxide reacts with another molecule of oxygen it breaks up again and releases chlorine which can again react with ozone and cause further depletion. Therefore adsorption air-conditioning technology attracted much attention recently as an alternative solution due to its advantage of environmental friendliness. That stratospheric ozone absorbs ultraviolet radiation that otherwise would reach the surface of Earth.
The Montreal Protocol has proven to be innovative and successful and is the first treaty to achieve universal ratification by all countries in the world. Substantial progress has been made in the ensuing years especially after the surprising discovery of the ozone hole in Antarctica in 1984. The initial step in the depletion of stratospheric ozone by human activities is the emission of ozone-depleting gases containing chlorine and bromine at Earths surface.
6 rows Chlorofluorocarbons. Chlorofluorocarbons or CFCs are the main cause of ozone layer. Chlorofluorocarbons CFCs and other halogenated ozone-depleting substances ODS are mainly responsible for man-made chemical ozone depletion.
The hole is actually an area of the stratosphere with extremely low concentrations of ozone that reoccurs every year at the beginning of the Southern Hemisphere spring August to October. Further work by Susan Solomon and colleagues clearly attributed the ozone hole to CFCs and other ozone-depleting chemicals that contained the elements chlorine and bromine.
Is Earth S Ozone Layer Still At Risk 5 Questions Answered

Causes And Effects Of Ozone Layer Depletion That Are Truly Dangerous Ozone Layer Ozone Ozone Layer Healing

Environment For Kids Ozone Layer Depletion مدونة
Ozone Depletion Images Stock Photos Vectors Shutterstock

10 2 Ozone Depletion Environmental Biology
Chlorofluorocarbons And Ozone Depletion American Chemical Society

How Cfc S Deplete The Ozone Layer Youtube
Antarctic Ozone Recovery Will Be A Long And Bumpy Road
What Is The Major Cause For Ozone Layer Thinning Or Depletion Quora

Ozone Depletion The Ozone Depletion Process Begins When Chloroflurocarbons Cfcs Also Ozone Depletion Ozone Layer Ozone
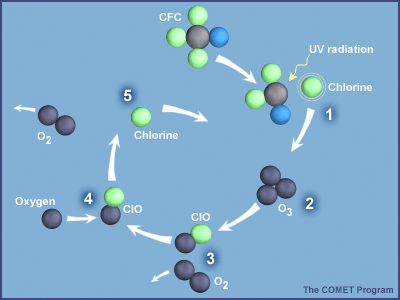
10 2 Ozone Depletion Environmental Biology

Depletion Of Ozone Layer And Its Effects Kofa Study

The Green Life World Ozone Day September 16 Ozone Layer Ozone Depletion Ozone

Pollution Thins Our Ozone Layer Ozone Depletion Ozone Layer Ozone
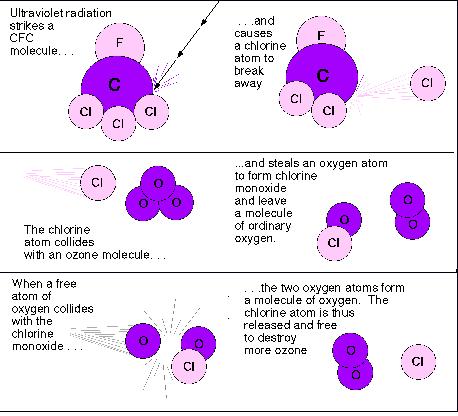

Comments
Post a Comment